New Delhi: On a day when Covishield vaccine prepared by Serum Institute of India rolled out from the Pune facility to 13 different locations, the Health Ministry informed that four more vaccines wherein different stages and would soon hit the market after due regulatory clearance. As of now, the government has allowed emergency authorisation to – Covisheild and Covaxin. However, the Union Health Secretary Rajesh Bhushan today announced that very soon vaccines from Zydus Cadila, Sputnik V, Biological E and Gennova would be in the Indian market.
Union Health Secretary, however, mentioned that these vaccines were in different stages of trial and approval will be given after due process.
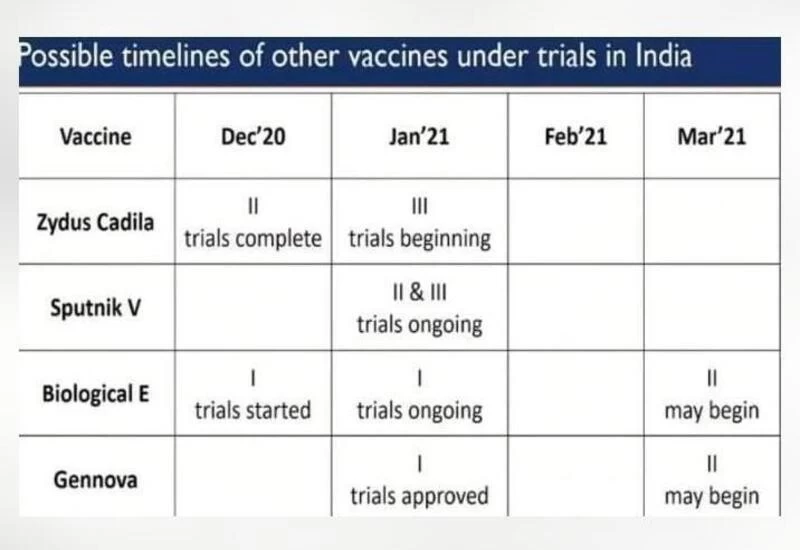
Zydus Cadila’s vaccine candidate is known as ZyCov-D and is being prepared in Ahmedabad. Sputnik V is the Russian vaccine which is being prepared in India by Dr Reddy’s Lab. Dr Reddy’s on Monday announced the beginning of Phase 3 trials in Hyderabad.
Biological E (BE) is currently working on the protein-based vaccine. The BE vaccine is being developed in collaboration with the Baylor College of Medicine, US.
The vaccine developed by Pune-based Gennova Pharmaceuticals has also entered the human trial stage. Gennova’s vaccine candidate is India’s first mRNA COVID-19 vaccine that can be stored at 2-8°C. End-user storage of vaccine which requires sub-zero temperature is being seen as the biggest challenge in India. Gennova’s vaccine therefore can come in handy for places where storage might be an issue.
4 New Vaccines Apart from Covishield and Covaxin in Pipeline
Zydus Cadila – ZyCov-D4 New Vaccines Apart from Covishield and Covaxin in Pipeline Zydus Cadila – ZyCov-D
Sputnik V – In Collaboration with Dr Reddy’s Lab
Biological E – In collaboration with Baylor College of Medicine, US
Gennova’s Vaccines – Indigenous COVID-19 vaccine based on the messenger RNA platform
Union Health Secretary also informed that the Government of India has agreed to procure 110 lakh Covishield vaccine doses from Serum Institute of India (SII) at Rs 200/dose.
55 lakh doses of Covaxin will be procured from Bharat Biotech (BBIL), of which 38.5 lakh doses priced at Rs 295/dose, Rajesh Bhushan said.
BBIL will provide 16.50 lakh doses of Covaxin free of cost to the Central govt as a special gesture, Bhushan said.